The ScreenPoint Transpara system is intended for use as a concurrent reading aid for radiologists during interpretation of 2D and 3D mammograms of asymptomatic women to identify regions suspicious for breast cancer. Output of the device contains region-based scores indicating the likelihood that cancer is present in specific regions and an overall score indicating the likelihood that cancer is present on the mammogram.
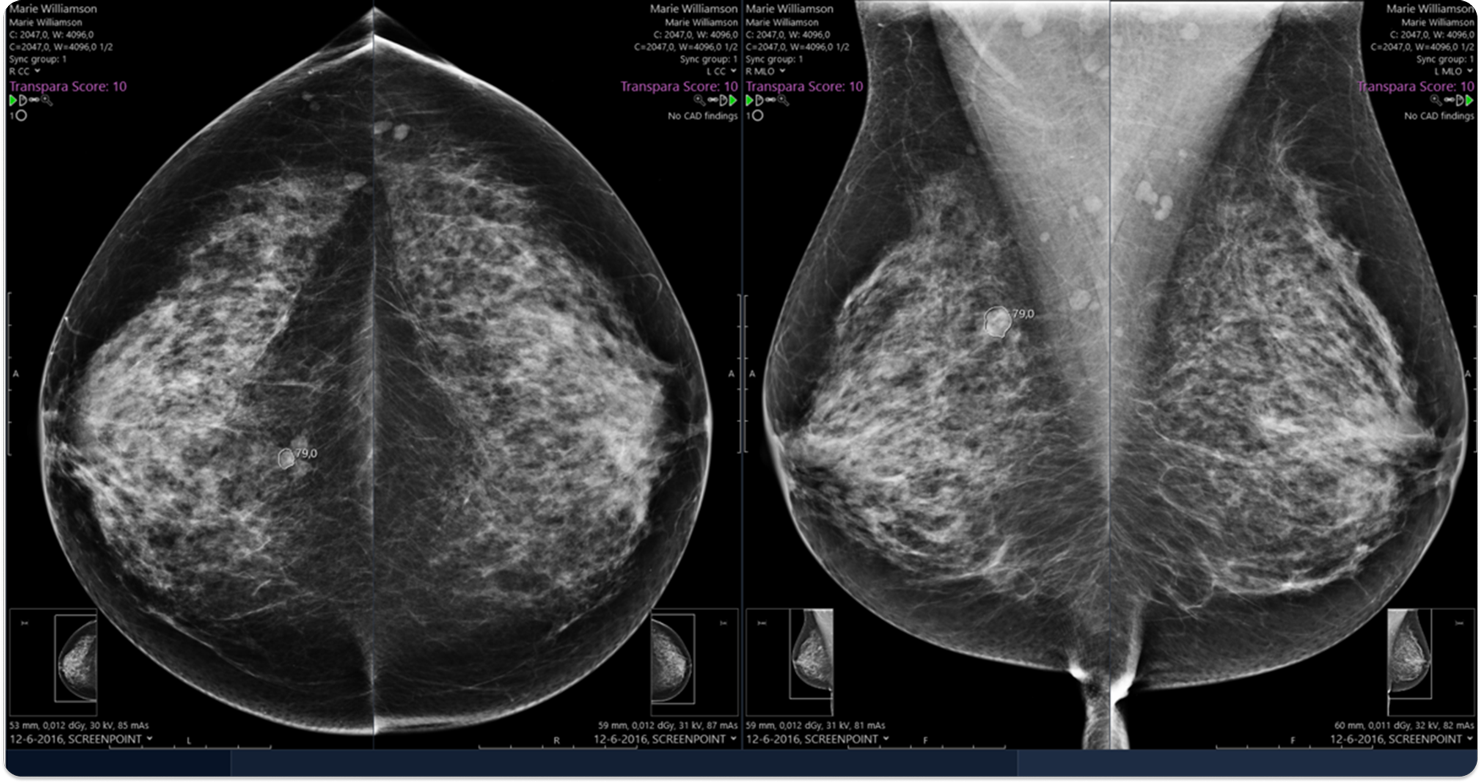
Images shown for illustrative purposes only.

Digital mammography and digital breast tomosynthesis (DBT, or 3D mammography).
Series: MLO, CC, ML and LM
Transpara offers the following functions:
- Computer aided detection (CAD) marks to highlight locations where the device detected suspicious calcifications or soft tissue lesions.
- Decision support is provided by region scores on a scale ranging from 1-100, with higher scores indicating a higher level of suspicion.
- Links between corresponding regions in different views of the breast, which may be utilized to enhance user interfaces and workflow.
- An exam based score which categorizes exams on a scale of 1-10 with increasing likelihood of cancer. The score is calibrated in such a way that approximately 10 percent of mammograms in a population of mammograms without cancer falls in each category.
Currently not made available with Calantic Viewer. Separately distributed by Bayer.
EU risk class and CE marking
Transpara has CE marking (CE0344) and risk class IIb.
Reimbursement status
Not reimbursed.
Contraindications
No contraindications given.
Target population
Asymptomatic women.
Intended user
Health care professionals such as a radiologist or radiographer.
Limitations
Transpara is a software device for automated detection of regions in mammograms suspicious for breast cancer. The device should not be used on its own. The final conclusion in the report of any mammographic exam processed by the device should always be made by a medical professional specialized in the reading of mammograms. Medical staff should not rely solely on the output of the device. Users should be aware that the system does not detect all cancers and that it regularly displays false positive findings not representing cancer.
To prevent misinterpretation of results the system should not be used without proper training. Review of the user manual is required prior to using the system. When the system is used as a perception aid, users should be aware that the system may detect a cancer only in one view while missing it in another view. When used as an interpretation aid, users should be aware that the device may occasionally assign a low abnormality score to a cancer or a high abnormality score to a normal region. Users should not rely solely on the device and always carefully inspect exams visually.
When the Transpara Score is used, users should be aware that the device may occasionally assign a low abnormality score to an abnormal exam or a high abnormality score to a normal exam. Users should never rely solely on the device and always carefully inspect exams visually.
Transpara effectiveness has only been established on MLO, CC, ML and LM views. Other view types will not be processed.
The software is not designed to detect abnormalities on stereotactic, spot compressed or other diagnostic/non-standard views, but may produce results if these views are not recognized and rejected for processing. Do not use Transpara results computed for unsupported views.
When the device is used as an interpretation aid in the interactive mode, it automatically links findings in MLO and CC views of the same breast. However, users should be aware that findings of the system in MLO and CC views may not be correctly linked or that a corresponding finding in the other view may not be detected by the system. The system delineates lesions and can mark individual calcifications. Users should be aware, however, that soft tissue lesions may not be accurately delineated by the system and that detected calcification groups may not include every individual calcification. Due to system failure or due to controlled deletion of studies (i.e. IOCM), Transpara results might not be available for a particular exam or for particular images in the exam. Verify, after installation, periodically and after every modification of the production environment, if the display device clearly indicates if processing results are available. The software is not designed to process views that contain implants, a pacemaker, or internal breast markers.
The device may not work properly if there are errors in DICOM tags (for example tags for image orientation, view, and laterality).
Images displayed on ScreenPoint Transpara Viewer are not of diagnostic quality and should only be used for reference with a workstation of diagnostic quality. Do not make a diagnosis based solely on the images displayed on the viewer.
User should check that the same patient’s exam is being shown in the Transpara Viewer and on the workstation used for primary assessment.
Markers displayed on synthetic images are computed on DBT volumes but the lesion might not be clearly visible or not visible in the synthetic. Assessment of the lesion pointed by the marker should be performed on the DBT slices. Digital Breast Tomosynthesis slice identification displayed on the Transpara Viewer may use a different numbering scheme than the diagnostic workstation. When referencing to a slice, never solely rely on the output of the device and always carefully inspect the slices in the volume.
If a study is not sent in send-after-close mode, and the study is read on Transpara Viewer soon after acquisition, it is possible that some images from the study are not visible and/or the Transpara Score does not take findings in the newest images into account. Per viewing device, only a single instance of the Viewer may be running at any time. The software’s effectiveness has not been established on mammograms of women with a history of breast cancer, mammograms with obvious signs of prior surgery, or women who are pregnant or lactating at the time of the mammography exam, or of males For DBT studies without synthetic images, Transpara Viewer should not be used to visualize Transpara results.
The software’s effectiveness has not been established for DBT volumes with strong reconstruction artefacts.


Discover Transpara
Now available as part of Calantic’s Breast Service Line. Contact us today to learn more.









